How an ORCHID will shape neuroscience, as published in Nature Communications.
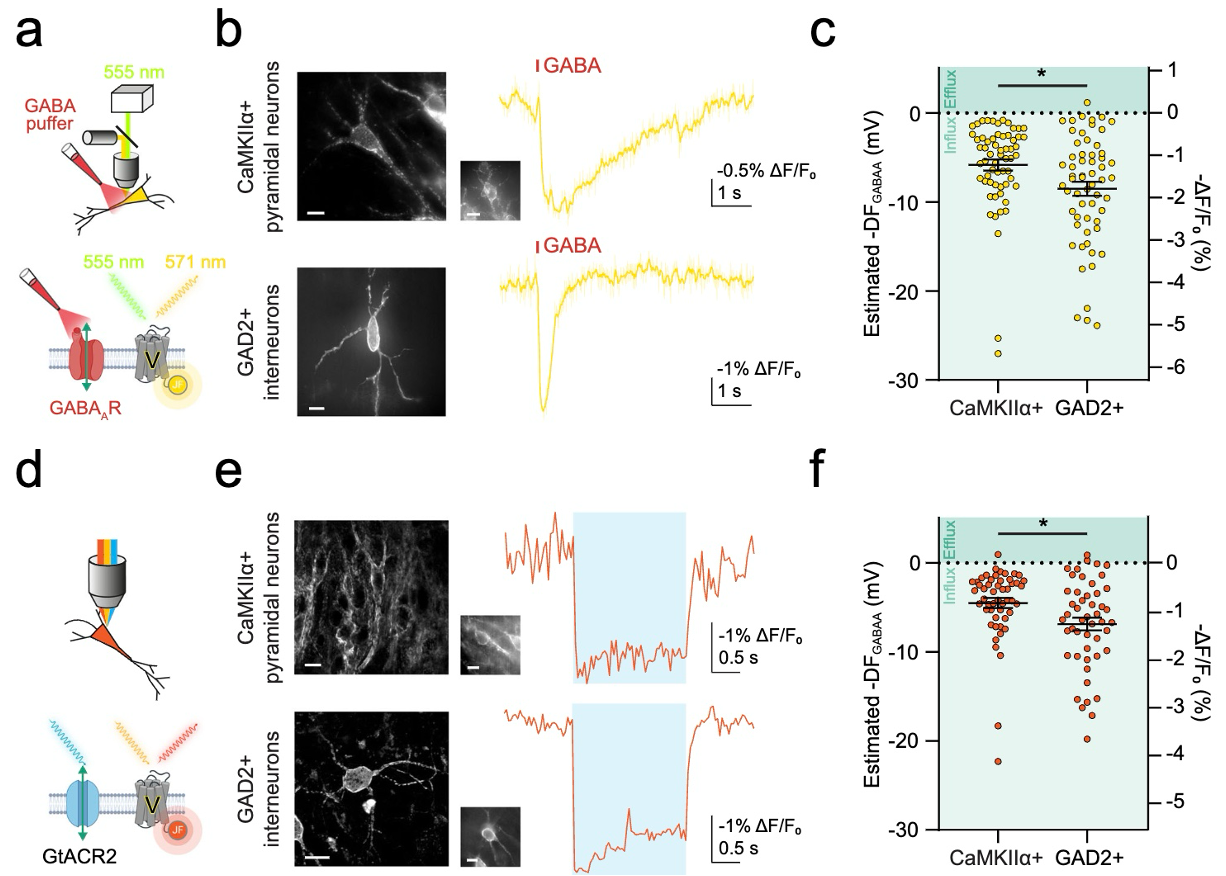
THE Nature Communications recently published a paper entitled: All-optical reporting of inhibitory receptor driving force in the nervous system by the Raimondo Lab and we couldn't be more excited (appropriately, this is the laboratory studying epilepsy). The paper is some hard-core electrophysiology, possibly indecipherable to most, so let us assure you, it is absolutely ground-breaking!
Here's as simplified as we can put it:
Chloride plays a critical role in our brains. Chloride ion movement across cell membranes is imperative to keep the brain’s inhibitory network functioning, which ultimately keeps all neural activity in check. This is because chloride ion movement ions flow through GABA receptors, which are the main inhibitory receptors so a problem with the flow of chloride ions can result in epileptic seizures and other significant neurological issues.
But measuring chloride ion movement has always been a challenge. Until recently this could only be done using invasive tools, directly sticking electrodes inside of single brain cells. The technical difficulty of this procedure meant data collection was limited and additionally the technique is disruptive to the cell which is further limiting, all of which has affected the depth of our understanding of the role of chloride in the brain.
This landmark research by Josh Selfe et al (in the Raimondo lab) has resulted in the development of a tool (ORCHID) which can accurately measure dynamic changes to the chloride receptor in the brain without disrupting the cells, simply by visualizing the cell and measuring changes in its fluorescence. This has wide-reaching applications for the world of discovery neuroscience.
This technique has several powerful advantages over currently available techniques, such as: leaving the cell unperturbed, allowing for more specific targeting in space and time, measuring changes orders of magnitude faster than before, and measuring cells that are difficult to access, all while being an incredibly stable method of recording. It can also be used in vivo and has the potential to record resting and dynamic changes to the chloride receptor throughout the mammalian brain, including from deep brain structures.
One could imagine combining ORCHID with other strategies to record from difficult to-access subcellular compartments, such as different parts of neurons and other brain cells. One could even measure chloride flux across the membranes of organelles, such as mitochondria, lysosomes, and vesicles. In doing so, ORCHID could be used to study a range of cellular phenomena including cell division, growth, and migration, in which chloride fluxes across different membranes contribute to the underlying intracellular and intercellular signalling processes.'
A massive congrats for this huge achievement to all the NI members and students who were involved: Joshua Selfe, Teresa Steyn, Eran Shorer, Richard Burman, Kira Düsterwald, Ariel Kraitzick and Joseph Raimondo!
This work was made possible by several grants, but the closest to home for us was the Gabriel Grants, enabled by the generosity of the NI's angel funders David and Ursel Barnes. This was one of nine projects generously funded by the Gabriel Foundation. Their seed funding has now grown, and in the case of ORCHID, truly bloomed.